Nasopharyngeal cancer is closely related to EBV infection, and China is a high incidence area, accounting for about half of the world's new cases every year. Early symptoms of nasopharyngeal cancer are not typical enough, and about 80% of patients are already in the middle or late stage when they are diagnosed, with poor treatment effect, short survival time and low quality of life. Screening by EBV double antibody (EBNA1-IgA+VCA-IgA) or two consecutive DNA tests can significantly improve the early diagnosis rate and survival rate of nasopharyngeal cancer patients, of which the double antibody screening program has been included in the "Early Diagnosis and Treatment of Cancer Program Technical Program" in 2011. However, only 4-5% of the high-risk patients screened by the dual antibody program were finally diagnosed with nasopharyngeal carcinoma after subsequent clinical examinations, and a large number of unnecessary clinical examinations (more than 95%) reduced the screening efficiency and increased the screening cost, and at the same time caused a serious psychological burden to the "false-positive" patients and their family members, resulting in a decrease in their compliance, which is difficult to be promoted on a large scale. This makes it difficult to promote on a large scale. Therefore, there is an urgent need to discover new screening markers for nasopharyngeal carcinoma to improve the screening efficiency.
Prof. Ningshao Xia's team systematically studied the anti-EBV antibody profile in the serum of nasopharyngeal carcinoma patients and healthy control subjects, and discovered a new serological marker for nasopharyngeal carcinoma: a total antibody recognizing the peptide encoded by the BNLF2b gene (abbreviated to as "P85-Ab"), and developed a high-throughput automated test kit together with Vantage Bio. From 2020 to 2021, a prospective cohort study of approximately 25,000 patients was conducted by Prof. Ningshao Xia's team and Prof. Mingfang Ji's team at Zhongshan People's Hospital in Zhongshan City, China, to compare head-to-head the efficacy of the P85-Ab test with that of the traditional double-antibody regimen for nasopharyngeal carcinoma (NPC) screening. A total of 47 cases of nasopharyngeal carcinoma were diagnosed in this study, and 38 cases were stage I/II cases. Compared with the double-antibody protocol, P85-Ab embodies two outstanding advantages:
1. higher sensitivity for early nasopharyngeal cancer. Among all nasopharyngeal cancers, the detection rates of P85-Ab and dual-antibody regimen were 97.9% and 72.3%, respectively, and the main difference was the sensitivity to early-stage nasopharyngeal cancers. 10 more stage I/II nasopharyngeal cancers were detected by P85-Ab compared with the dual-antibody regimen (37 vs. 27), and the early diagnosis rate was increased by 22% (79% vs. 57%);
2. higher specificity, with a more than 2-fold increase in positive predictive value compared to dual-antibody screening (10.0% vs. 4.3%).
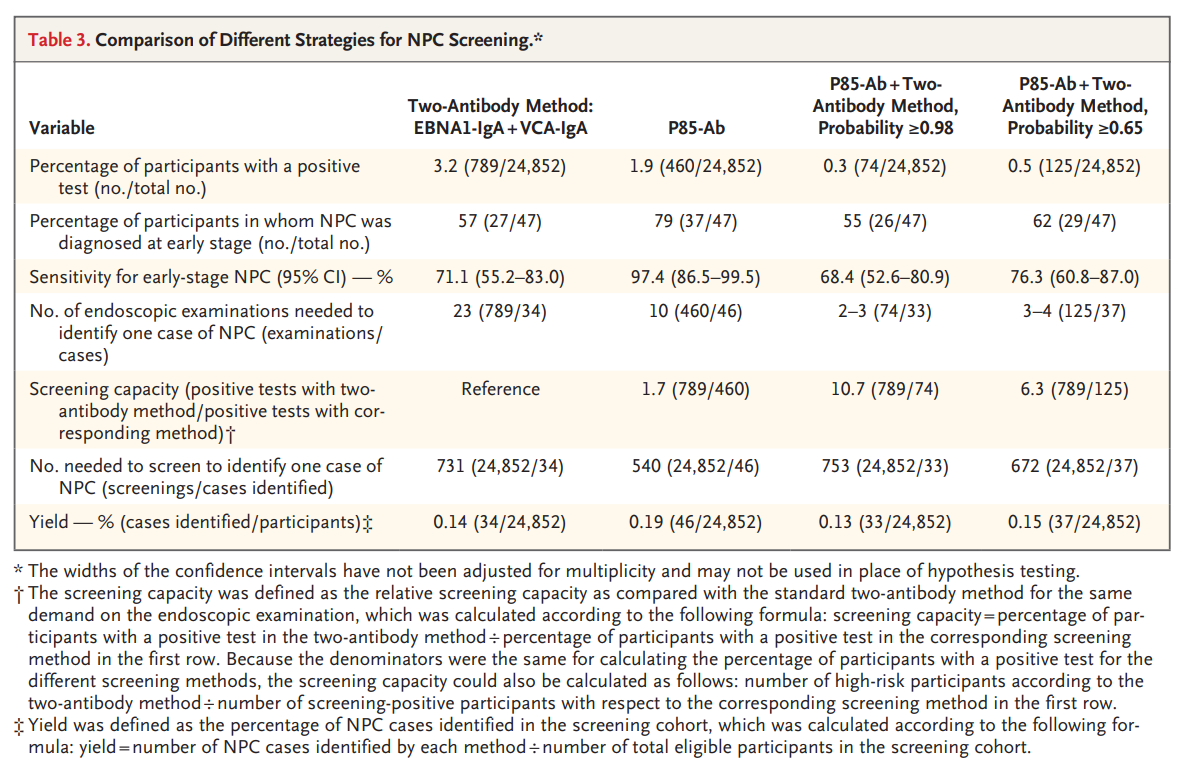
P85-Ab screening was also found to be complementary to the dual antibody screening program. On the basis of P85-Ab positivity, further risk stratification by the dual antibody protocol can increase the positive predictive value of nasopharyngeal cancer screening to 29.6%~44.6%, i.e., one nasopharyngeal cancer can be diagnosed for every 2~3 nasopharyngoscopies, which can significantly improve the efficiency of nasopharyngeal cancer screening. Without increasing the number of nasopharyngoscopy, the screening scale of combined screening can be increased by 6~11 times compared with the original double antibody screening program.
As a new nasopharyngeal cancer marker with higher sensitivity, specificity and positive predictive value, P85-Ab can significantly improve the efficacy, cost-effectiveness and acceptability of nasopharyngeal cancer screening, which will help to expand the coverage of nasopharyngeal cancer screening, improve the early diagnosis and treatment rate of nasopharyngeal cancer, and ultimately reduce the disease burden of nasopharyngeal cancer.
Recently, the research results were published online in The New England Journal of Medicine as "Anti-Epstein-Barr Virus BNLF2b for Mass Screening of Nasopharyngeal Cancer". England Journal of Medicine.) Tingdong Li, Senior Engineer of National Engineering Center, Xiaoyi Guo, Graduate Doctoral Student of National Engineering Center, Hong Congming, Engineer of National Engineering Center, and Fugui Li, Chief Technician of Zhongshan People's Hospital, and Xia Yu, Attending Physician of Zhongshan People's Hospital are the co-first authors of the article. Prof. Ningshao Xia, Prof. Shengxiang Ge of the National Engineering Center and Prof. Mingfang Ji of Zhongshan People's Hospital are the co-corresponding authors of the article. The study was supported by the National Key Research and Development Program, the Basic Research Operating Expenses of the Central Universities, the Fujian Provincial Science and Technology Major Project, the National Natural Science Foundation of China, as well as the long-term platform support given by the Ministry of Science and Technology, the Ministry of Education, the National Healthcare Commission, the National Development and Reform Commission, the State Administration of Pharmaceutical Administration, the Fujian Provincial Government, and the Xiamen Municipal Government.